Tetracobalt dodecacarbonyl
| |
| Names | |
|---|---|
| Other names
cobalt dodecacarbonyl, cobalt carbonyl
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.037.951 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Co4(CO)12 | |
| Molar mass | 571.858 g/mol |
| Appearance | black crystal |
| Density | 2.09 g/cm3 |
| Melting point | decomposes at 60 °C (140 °F; 333 K) |
| Hazards | |
| GHS labelling: | |
  
| |
| Warning | |
| H228, H301, H317, H331, H351 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
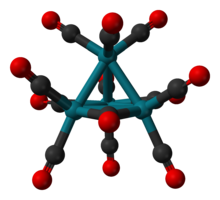
Tetracobalt dodecacarbonyl is the chemical compound with the formula Co4(CO)12. It is a black crystalline compound that is insoluble in water and easily oxidized by air. It is an example of a metal carbonyl cluster.
Synthesis and structure
[edit]This compound is synthesized by decarbonylation of Co2(CO)8.
- 2 Co2(CO)8 → Co4(CO)12 + 4 CO
The molecule consists of a tetrahedral Co4 core, but the molecular symmetry is C3v. Three carbonyl ligands are bridging ligands and nine are terminal.[1] The average Co-Co distance is 2.499 Å, the average C-O bond length is 1.133 Å, and the average Co-C-O angle is 177.5°.[2][3]
Rh4(CO)12 adopts the same C3v structure but Ir4(CO)12 has perfect Td symmetry with no bridging CO ligands groups.[4] The Rh4 and Ir4 clusters are more thermally robust than that of the Co4 compound, reflecting the usual trend in the strengths of metal-metal bond for second and third row metals vs those for the first row metals. There has been disagreement between the theoretically predicted and experimental structure of tetracobalt dodecacarbonyl.[5][4][6]
References
[edit]- ^ Chini, P. (1968). "The closed metal carbonyl clusters". Inorganica Chimica Acta. 2: 31–51. doi:10.1016/0073-8085(68)80013-0.
- ^ Farrugia, L. J.; Braga, D.; Grepioni, F. (1999). "A structure redetermination of Co4(CO)12: evidence for dynamic disorder and the pathway of metal atom migration in the crystalline phase". Journal of Organometallic Chemistry. 573 (1–2): 60–66. doi:10.1016/S0022-328X(98)00879-1.
- ^ Corradini, P. (1959). "Structure of tetracobaltdodecarbonyl". Journal of Chemical Physics. 31 (6): 1676–1677. Bibcode:1959JChPh..31.1676C. doi:10.1063/1.1730674.
- ^ a b Wei, C. H. (1969). "Structural analyses of tetracobalt dodecacarbonyl and tetrarhodium dodecacarbonyl. Crystallographic treatments of a disordered structure and a twinned composite". Inorganic Chemistry. 8 (11): 2384–2397. doi:10.1021/ic50081a030.
- ^ Corradini, Paolo (1959). "Structure of tetracobaltdodecarbonyl". Journal of Chemical Physics. 31 (6): 1676–1677. Bibcode:1959JChPh..31.1676C. doi:10.1063/1.1730674.
- ^ Farrugia, Louis J.; Braga, Dario; Grepioni, Fabrizia (1999). "A structure redetermination of Co4(CO)12: Evidence for dynamic disorder and the pathway of metal atom migration in the crystalline phase". Journal of Organometallic Chemistry. 573 (1–2): 60–66. doi:10.1016/S0022-328X(98)00879-1.
